The Clinical CRISPR Successes Are Stacking Up. But Where’s the Emphasis on Quality Control?
In mid-September, Intellia released some promising data on two of their CRISPR-based therapeutics. These results are a significant milestone for genome editing and validation of its effectiveness in the clinic.
However, the safety of CRISPR-based therapeutics has been a major concern and part of a larger conversation about mitigating risk throughout genome editing drug discovery and clinical development.
Here we discuss some of the CRISPR world’s clinical victories and lingering concerns, including:
- Intellia’s One-Time Treatment for Hereditary Angioedema (HAE)
- More Clinical Results from Intellia
- Emerging Safety Issues with Genome Editing
- How Can Safety Challenges with CRISPR be Addressed?
- Defining an Analytics Platform for Derisking Genome Editing Therapies
- References
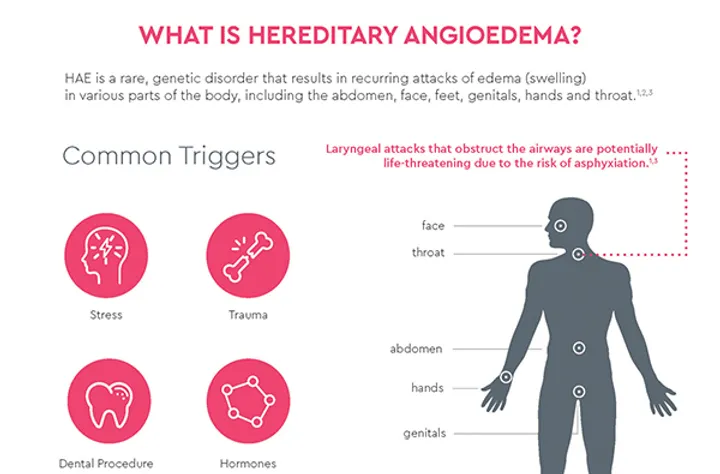
Intellia’s NTLA-2002 Trial: A One-Time Treatment for Hereditary Angioedema (HAE)
An interim readout from the company’s phase I/II trial, testing drug candidate NTLA-2002, demonstrated a reduced occurrence of swelling attacks by 91% in patients with a rare condition called hereditary angioedema (HAE).1
HAE is a fatal genetic disease that can cause painful and fatal swelling in patients. To treat HAE, Intellia’s therapy targets and disrupts the gene KLKB1, which encodes for prekallikrein, a precursor to the kallikrein protein involved in blood clotting. Treatment with NTLA-2002 reduced the concentration of kallikrein in both low- and high-dose patient cohorts by 65% and 92%, respectively.1
These results bring the hope of a one-time treatment for those that suffer from the broader patient population living with the debilitating symptoms of HAE.
More Clinical Results from Intellia
NTLA-2002 is the second clinical trial and genome editing success reported by Intellia. Their first therapeutic agent to enter the clinic, NTLA-2001, successfully targets a mutated version of the gene TTR, which causes a destructive hereditary disease called transthyretin (ATTR) amyloidosis due to the buildup of misfolded transthyretin (TTR) in the nerves (which can cause polyneuropathy) and heart (which can cause cardiomyopathy).
Treatment of patients with AATR amyloidosis with polyneuropathy using NTLA-2001 resulted in 52% and 87% reduction in TTR in the low- and high-dose treatment cohorts, reported in mid-2021.2
Intellia’s most recent announcement in September of 2022 reported that their AATR amyloidosis with cardiomyopathy cohort (with 12 participants) experienced a 92 to 94% reduction in mutated TTR when treated with NTLA-2001.1
NTLA-2001 and NTLA-2002 are lipid nanoparticle-encapsulated CRISPR-Cas systems that deliver their genome editing payload to the liver. These promising results validate this approach as an effective method for correcting disease-causing mutations in humans.
Emerging Safety Issues with Genome Editing
Despite these promising outcomes and assurances that there were no reported liver toxicities or serious treatment-related adverse events, news outlets (here and here) documented elevated liver enzyme levels in one patient in the AATR amyloidosis with polyneuropathy arm of Intellia’s NTLA-2001 trial.3,4 Though it’s still unclear whether the elevated liver enzymes are related to NTLA-2001 treatment, this reality has led Intellia to amend its clinical protocol, including a fixed low-dose arm.4 Though the patient was asymptomatic and their medical condition was deemed “nonserious,” the news led to a significant downturn in Intellia stock and the stock of other CRISPR therapeutics companies.3
Elevated liver enzymes are not the first safety concern raised with genome editing therapies. In early 2021, bluebird bio halted its sickle cell trial after two patients developed leukemia-like cancer.5
Of course, safety concerns are typical in first-in-human studies with experimental treatments and are carefully monitored during any clinical trial. Yet, since the discovery that CRISPR-Cas genome editing could be harnessed to permanently make genetic changes in humans, ethical and safety worries have swirled, sometimes clouding the promise of this exciting therapeutic approach.6,7
How Can Safety Challenges with CRISPR be Addressed?
As more and more genome editing technologies are developed and enter the clinic, regulatory agencies and public health organizations, such as the WHO, have provided recommendations and guidelines to help drug developers focus on safety, effectiveness, and ethics.8
Guidance from the FDA and EMA and initiatives from the National Institute of Standards and Technology (NIST) are emphasizing the need for standardization during the discovery and development processes for CRISPR-Cas therapeutics.
Ultimately, these regulatory standards are still shifting.
Yet, their focus on derisking clinical candidates before they get to human trials through the implementation of quality control and standardized processes and optimizing in vitro and in vivo assays is a major step in the right direction to preventing future safety concerns.
Defining an Analytics Platform for Derisking Genome Editing Therapies
As more clinical trials hit the ground, it’s important to continue emphasizing quality control and standardization in CRISPR editing. Putting this into practice, however, requires precision tools that provide actionable data.
At CRISPR QC, we’re working to empower the scientists, researchers, and doctors at the front lines of the genetic engineering revolution with the data and insights they need to power the safest, most efficacious gene editing outcomes for patients. Using our end-to-end analytics platform, we help you uncover the causal factors behind genome editing success or failure. The insights extracted from simulating the editing process in vitro lead to better editing downstream.
Contact us to learn more about our CRISPR-focused analytics platform and to see it in action.
References
- Intellia says CRISPR treatment safely corrects DNA of six patients with rare disease. STAT website: https://www.statnews.com/2022/09/16/intellia-says-crispr-treatment-safely-corrects-dna-of-six-patients-with-rare-disease/. Accessed October 19, 2022. Published September 16, 2022.
- Gillmore JD, Gane E, Taubel J, et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N Engl J Med. 2021;385(6):493-502. doi:10.1056/NEJMoa2107454
- Intellia, Other CRISPR Stocks Hammered On Safety Concerns In New Gene-Editing Test. Investors Business Daily website: https://www.investors.com/news/technology/crispr-stock-will-intellia-latest-gene-editing-effort-buoy-the-market-again/. Accessed October 19, 2022. Published September 16, 2022.
- Intellia tacks on lower dose to polyneuropathy trial arm after elevated liver level in one patient. Fierce Biotech website: https://www.fiercebiotech.com/biotech/intellia-looks-tack-lower-dose-polyneuropathy-trial-arm-after-elevated-liver-levels. Accessed October 19, 2022. Published August 4, 2022.
- Gene therapy trials for sickle cell disease halted after two patients develop cancer. Science website: https://www.science.org/content/article/gene-therapy-trials-sickle-cell-disease-halted-after-two-patients-develop-cancer. Accessed October 19, 2022. Published February 16, 2021.
- The CRISPR-baby scandal: what’s next for human gene-editing. Nature website: https://www.nature.com/articles/d41586-019-00673-1. Accessed October 19, 2022. Published March 11, 2019.
- Ishii T, Beriain IM. Safety of Germline Genome Editing for Genetically Related “Future” Children as Perceived by Parents. CRISPR J. 2019;2(6):370-375. doi:10.1089/crispr.2019.0010